Toluenesulfonic Acid on:
[Wikipedia]
[Google]
[Amazon]
''p''-Toluenesulfonic acid (PTSA or ''p''TsOH) or tosylic acid (TsOH) is an
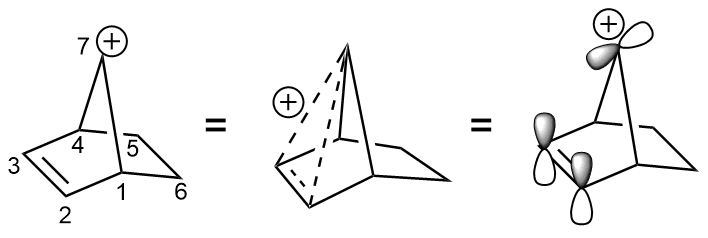 In a famous and illustrative use of tosylate, 2-norbornyl cation was displaced from the 7-norbornenyl tosylate. The elimination occurs 1011 times faster than the solvolysis of ''anti''-7-norbornyl ''p''-toluenesulfonate.
Tosylates are also protecting group for
In a famous and illustrative use of tosylate, 2-norbornyl cation was displaced from the 7-norbornenyl tosylate. The elimination occurs 1011 times faster than the solvolysis of ''anti''-7-norbornyl ''p''-toluenesulfonate.
Tosylates are also protecting group for
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
with the formula CH3 C6H4 SO3H. It is a white extremely hygroscopic solid that is soluble in water, alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s, and other polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
organic solvents. The CH3C6H4SO2 group is known as the tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a Toluene, tolyl group, –, joined to a sulfonyl group, ––, with the open vale ...
group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was underst ...
, TsOH.H2O.
As with other aryl sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
s, TsOH is a strong organic acid. It is about one million times stronger than benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
. It is one of the few strong acids that is solid and therefore is conveniently weighed and stored.
Preparation and uses
TsOH is prepared on an industrial scale by thesulfonation Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution. Aryl sulfonic acids are used as detergents, dye, and drugs.
Stoichiometry a ...
of toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
. Common impurities include benzenesulfonic acid
Benzenesulfonic acid (conjugate base benzenesulfonate) is an organosulfur compound with the formula C6 H6 O3 S. It is the simplest aromatic sulfonic acid. It forms white deliquescent sheet crystals or a white waxy solid that is soluble in water ...
and sulfuric acid. TsOH monohydrate contains an amount of water. To estimate the total moisture present as impurity, the Karl Fischer method is used. Impurities can be removed by recrystallization from its concentrated aqueous solution followed by azeotropic
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
drying with toluene.
TsOH finds use in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
as an "organic-soluble" strong acid. Examples of uses include:
*Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...
ization of an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
.
*Fischer–Speier esterification
Fischer esterification or Fischer–Speier esterification is a special type of esterification by refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction was first described by Emil Fischer and Arthur Speier ...
*Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction can ...
reactions
Tosylates
Alkyl tosylates arealkylating agent
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
s because tosylate is electron-withdrawing
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of th ...
as well as a good leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
. Tosylate is a pseudohalide. Toluenesulfonate esters undergo nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
or elimination. Reduction of tosylate esters gives the hydrocarbon. Thus, tosylation followed by reduction allows for the deoxygenation of alcohols.
 In a famous and illustrative use of tosylate, 2-norbornyl cation was displaced from the 7-norbornenyl tosylate. The elimination occurs 1011 times faster than the solvolysis of ''anti''-7-norbornyl ''p''-toluenesulfonate.
Tosylates are also protecting group for
In a famous and illustrative use of tosylate, 2-norbornyl cation was displaced from the 7-norbornenyl tosylate. The elimination occurs 1011 times faster than the solvolysis of ''anti''-7-norbornyl ''p''-toluenesulfonate.
Tosylates are also protecting group for alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s. They are prepared by combining the alcohol with 4-toluenesulfonyl chloride, usually in an aprotic solvent, often pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
.
Reactions
* TsOH may be converted to ''p''-toluenesulfonic anhydride by heating withphosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4 O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydra ...
.
* When heated with acid and water, TsOH undergoes hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
to toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
:
:CH3C6H4SO3H + H2O → C6H5CH3 + H2SO4
This reaction is general for aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
s.
See also
*Tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a Toluene, tolyl group, –, joined to a sulfonyl group, ––, with the open vale ...
* Collidinium ''p''-toluenesulfonate
References
{{DEFAULTSORT:Toluenesulfonic Acid, P- Benzenesulfonic acids Reagents for organic chemistry Acid catalysts Sulfonic acids P-Tosyl compounds